To understand how neural activity changes with learning and aging, or to study neurological disorders, it is important to develop long-term stable recordings of the same neurons at single-cell and single-spike resolution.
Because current implantable devices cause immune responses and recording drift caused by disparities between these devices and brain tissue, it is necessary to address these challenges and develop a new method for implantation.
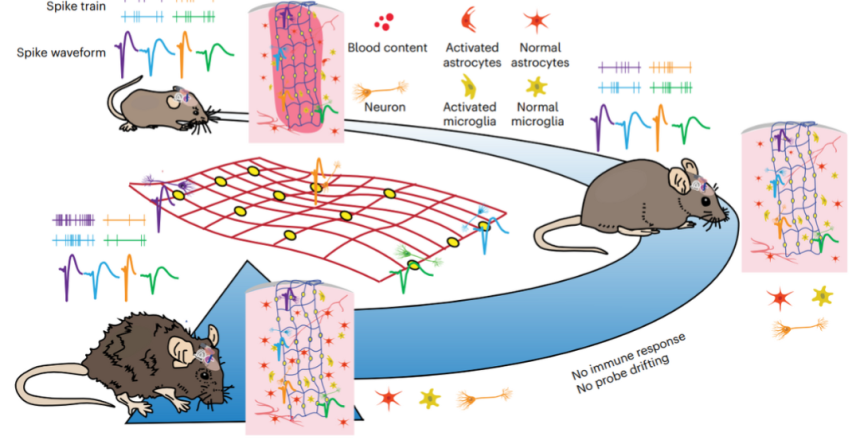
Article titled: “Tracking neural activity from the same cells during the entire adult life of mice” presents a new device that can track neural activity of the same cells throughout the adult life of mice. Authors Siyuan Zhao et al. developed a method for implanting fully unfolded tissue-like mesh electronics into the mouse brain that forms an interwoven structure within the neural network and eliminates immune responses, probe drift and neuron loss.
To fully implant an open and unfolded mesh structure into the brain, the mesh electronics were connected with ultra-thin, detachable polymeric shuttles. The mesh electronics were connected to the polymer shuttles with a PEG/Dextran layer, and detached from these shuttles by dissolving PEG in an aqueous solution.
The unfolded mesh structure interwove with the neural network and showed little to no inflammation and mechanical damage to the surrounding tissues during one year of implantation compared with the thin-film electronics.
Statistical analysis, visual stimulus-dependent measurements and unbiased, machine-learning-based analyzes demonstrated the capability of this device to:
- Stable record across multiple brain region;
- Stable track the same single-unit action potentials;
- Track behavior-dependent activities from the same neurons;
- Track the same neuron’s activity over the adult life of mice; and
- Long-term study the brain aging at the single-neuron level.
This technology has the potential to reveal long-term neural processes, such as development, learning, recovery from injury, neurodegeneration and age-related cognitive decline. It may also open new opportunities for neuroscience studies, next-generation brain–machine interfaces and bioelectronic medicine.
For more information read the full paper here and check out the video summary on our Instagram page.